Pneumococcus antisera overview
Pneumococcus, or Streptococcus pneumoniae, is a bacterium that can cause a range of illnesses—from mild respiratory infections and otitis media to life-threatening diseases such as pneumonia, meningitis, and sepsis. Serotyping is essential for understanding the epidemiology, virulence, and vaccine coverage of pneumococcal strains, as different serotypes vary in prevalence, invasiveness, and resistance profiles.
Pneumococci are enclosed within a capsule consisting of repeated units of polysaccharides. The composition of these
polysaccharides defines the respective serotype. More than 92 pneumococcal serotypes have been described, although not all exhibit distinct phenotypic profiles detectable by conventional serotyping methods. Serotyping of pneumococcus is typically performed with either the gold-standard Neufeld (Quellung) reaction or with latex agglutination (ImmuLex™)
using highly specific polyclonal antisera or antibodies that selectively bind to specific pneumococcal serotypes.
Neufeld vs. agglutination method comparison
| Neufeld (Quellung) | Agglutination (ImmuLex™) | |
|---|---|---|
| Product specification | Purified antisera (CE IVD) | Purified antisera conjugated to blue latex particles (CE IVD) |
| Primary use | Gold‑standard capsular reaction; full serotyping with pool/type/factor antisera | Rapid confirmation; Serogrouping; Partial serotyping on cards |
| Microscope needed | yes | no |
| Typical tests per unit | ~300 tests (1 mL vial) | ~75 tests (1.5 mL vial) |
| Key products | Omni / Pools / Groups / Types / Factor antisera; Pneumotest kit; High titer antisera; CWPS antiserum | ImmuLex™ Omni; ImmuLex™ latex pools / types / factors; ImmuLex™ Pneumotest kit |
Pneumococcus antisera solutions
Pneumococcus antisera for Neufeld
Omni, pool, group, type and factor antisera.
Pneumococcus high titer antisera
May also be used for non-diagnostic purposes like QC testing of multivalent pneumococcal conjugate and polysaccharide vaccines.
Anti CWPS serum
CWPS antiserum for Neufeld
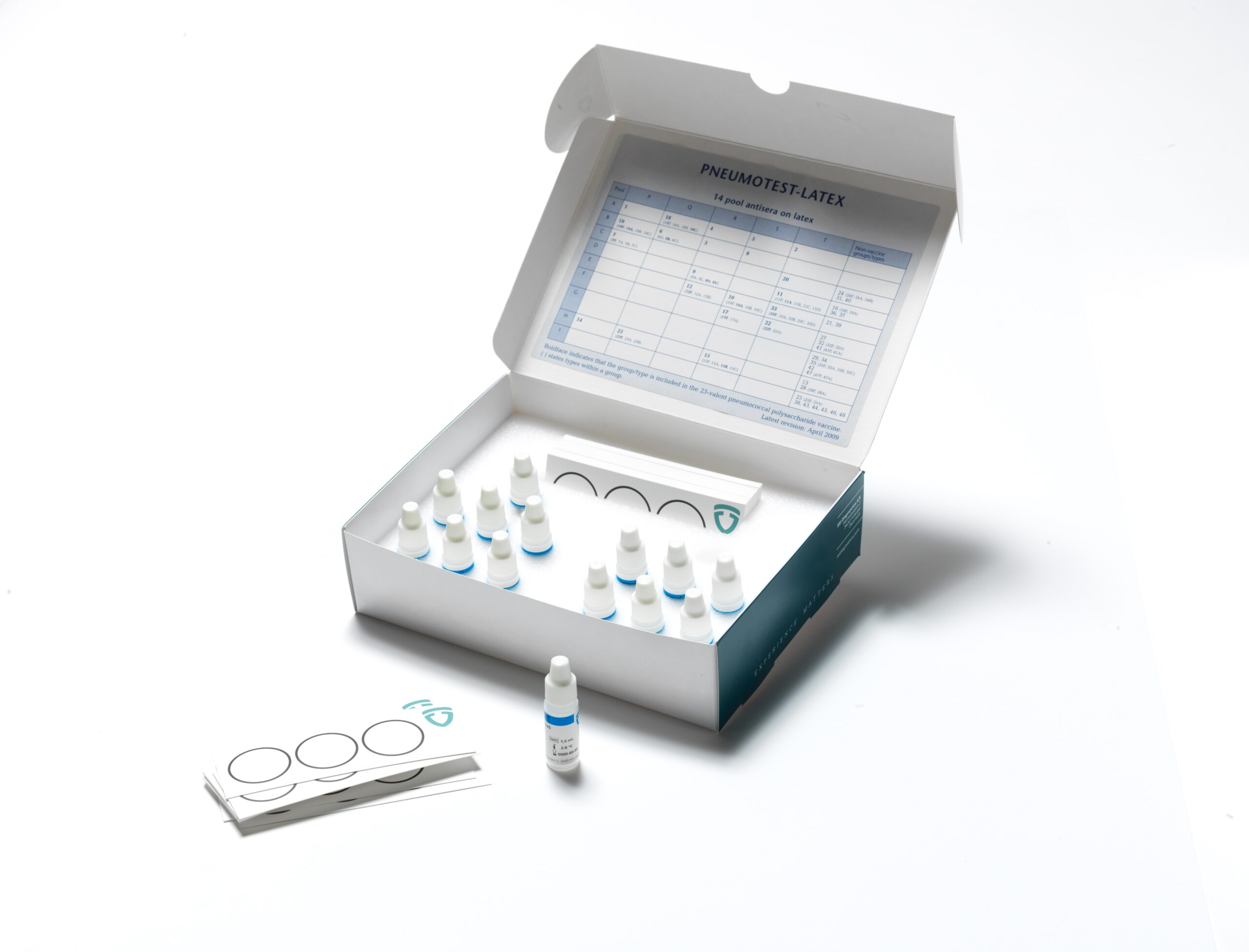
ImmuLex™ Pneumococcus
products
Pneumococcus latex products
for serotyping based on agglutination.
ImmuLex™ S. pneumoniae
Omni kit
For qualitative detection of S. pneumoniae directly from a positive blood culture or pure isolate.
Clinical and pharmacological relevance
Serotyping of pneumococci is used for surveillance of serotype distribution in connection with pneumococcal vaccination of both children and adults. Pneumococcal antisera from SSI Diagnostica Group are intended for visual qualitative confirmation and serotyping in clinical microbiological laboratories and reference laboratories. In the pharmaceutical industry, HT antisera are used in quality control assays of pneumococcal vaccines.
Quality, shelf life and compliance
All antisera are polyclonal and produced by immunizing rabbits with well-defined proprietary reference strains.
SSI Diagnostica Group’s antisera products are CE-marked and produced in accordance with DS/EN ISO 13485. The shelf life is 4 years from the date of production, and a minimum of 2 years from shipping.
Get in touch
If you have any questions, comments, or collaboration inquiries, you are very welcome to reach out.
Please fill in the form and we will get back to you as soon as possible.